Why are atomic spectra of an element discontinuous?
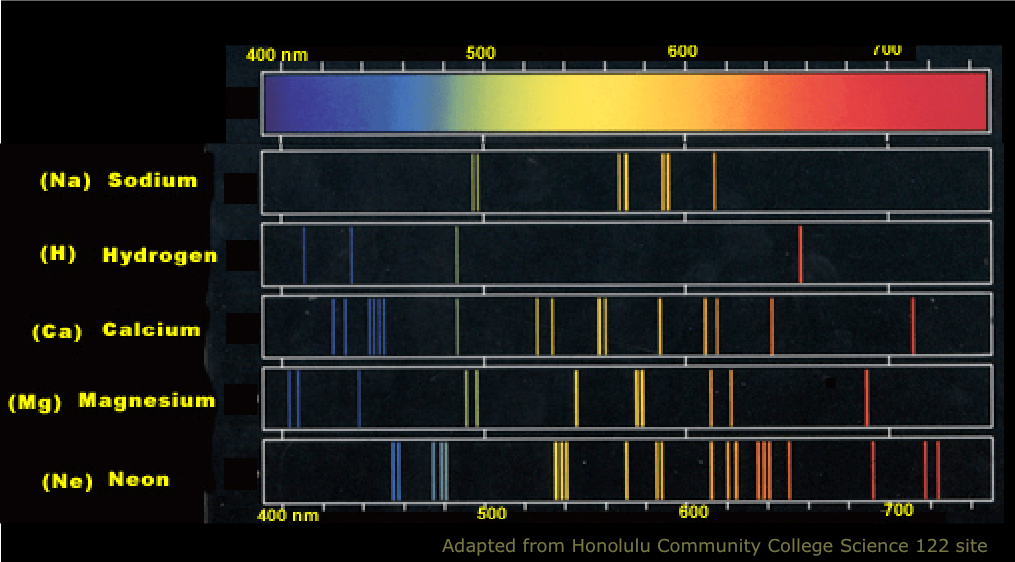
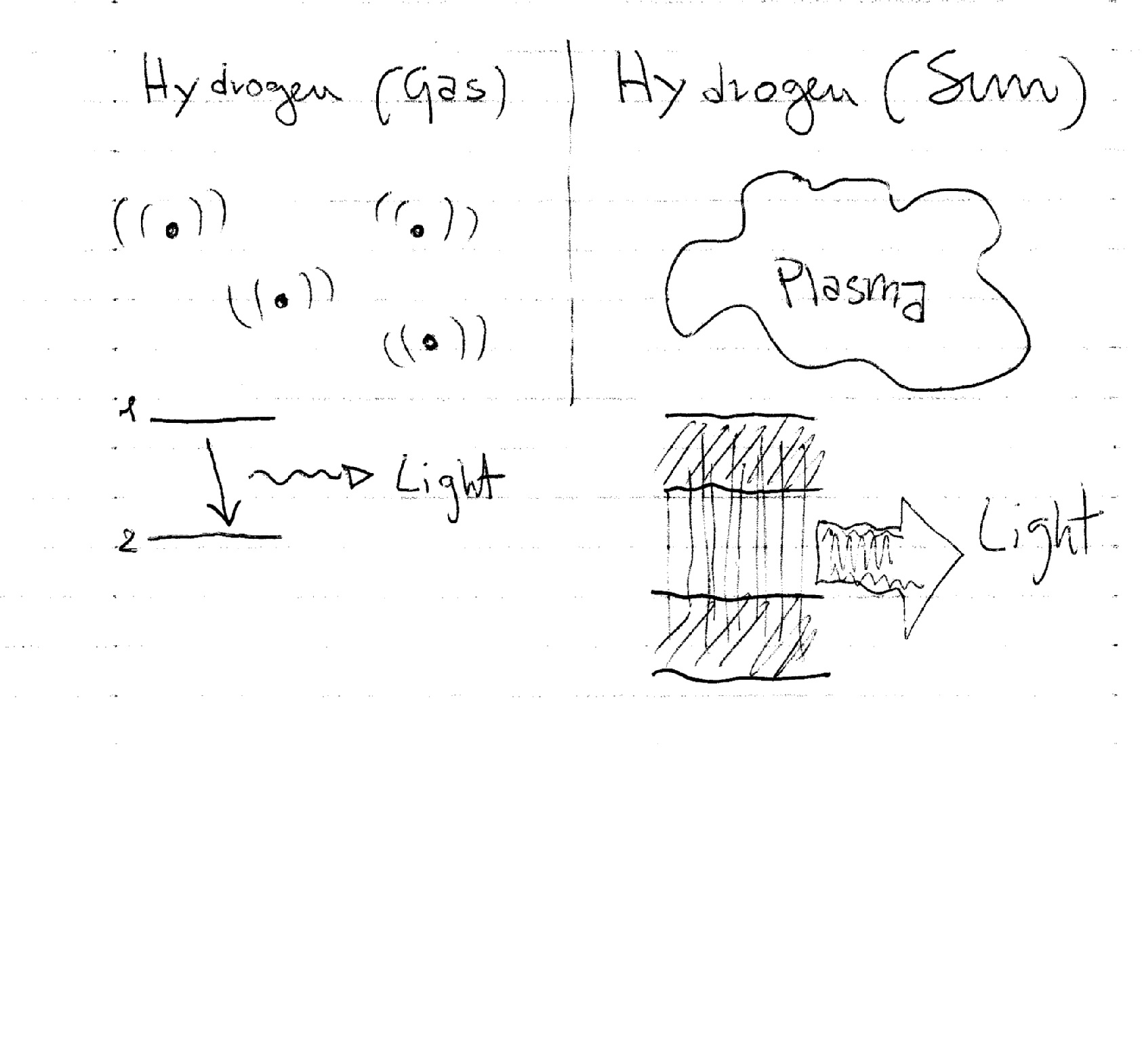
Quick answer: Atomic spectra are continuous because the energy levels of electrons in atoms are quantized. The electrons in an atom can have only certain energy levels. There is no middle ground. If an electron is excited to a new energy level, it jumps to that level instantaneously. When it returns to a lower level, it releases energy in a quantized packet. This release occurs in the form of light of a specific wavelength (colour). Hence, atomic emission spectra represent the electrons returning to lower energy levels. Each packet of energy corresponds to a line in the atomic spectrum. There is nothing between each line, so the spectrum is discontinuous.
Question #20ecb
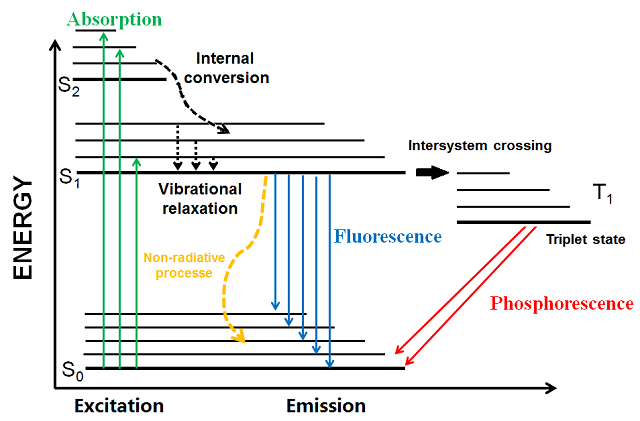
When an electron jumps from a high energy state to a lower state, what form does the emitted energy take?
4.2: Understanding Atomic Spectra - Chemistry LibreTexts

Spectrum ( absorption and emission, continuous and discontinuous) --Structure of atom lesson 6
How does an atomic spectrum get affected by external electromagnetic fields?
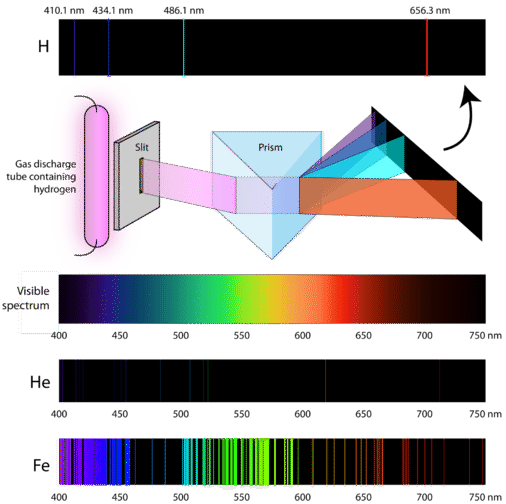
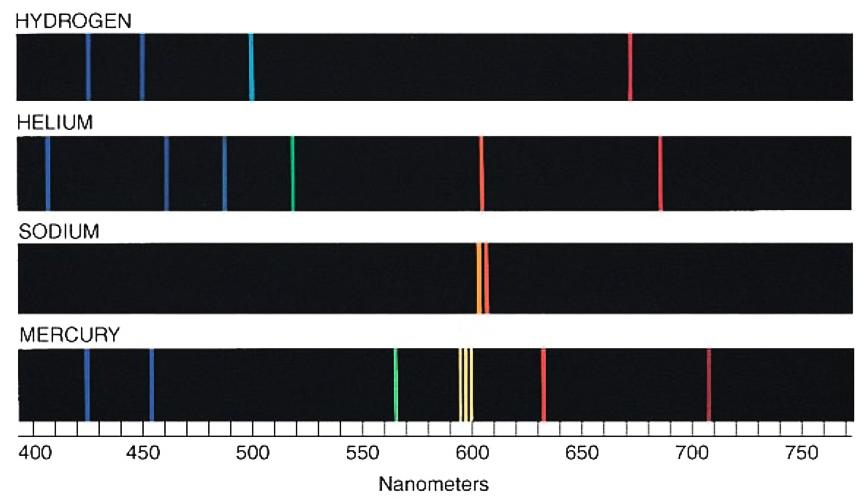
What are the spectral series we see in the Hydrogen atom emission spectrum?
Why is the spectrum of helium different from that of hydrogen?
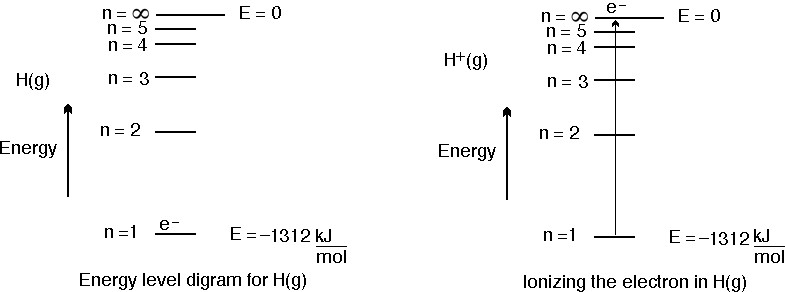
How do you calculate the ionization energy of a hydrogen atom in its ground state?

Notes On Atomic Spectra - CBSE Class 11 Chemistry
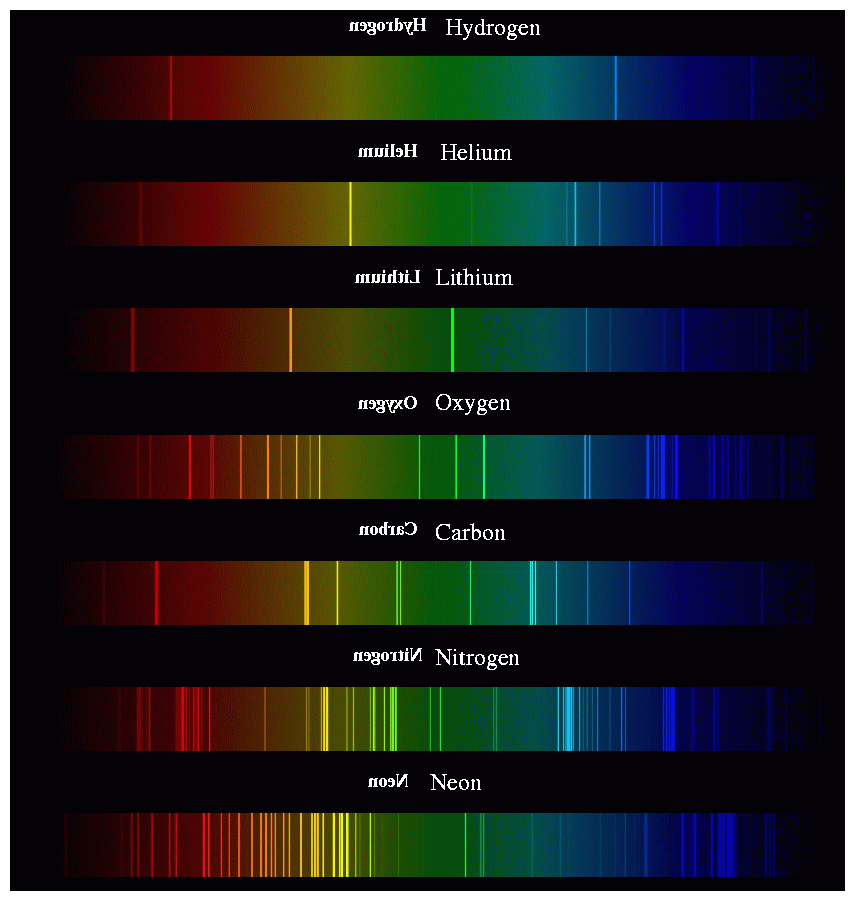
Using diffraction gratings to identify elements

Question #ce8f3