ORGANIC POLYMERS: THE SYNTHESIS OF NYLON - ppt video online download

Objectives: To synthesize Nylon 6,10 from hexamethylene diamine and sebacoyl chloride. To determine the length of the Nylon formed using a simple mathematical calculation.
ORGANIC POLYMERS: THE SYNTHESIS OF NYLON
Experiment 25: ORGANIC POLYMERS: THE SYNTHESIS OF NYLON.
Reacting a carboxylic acid with an amine yields an amide. Water is a by-product, and this reaction is slow without a catalyst.
By using a carboxylic acid chloride, a more reactive carboxylic acid derivative, the rate of reaction can be increased. In this reaction, HCl is the by-product.
SYNTHESIS OF NYLON 6,10. Sebacoyl chloride. Hexamethylene diamine. In order to make a polyamide, such as Nylon 6,10, the amine molecule must have a –NH2 group at each end, and the acid chloride must have a –COCl group at each end. The diamine and the diacid chloride bond together, end-on-end, to form very long chains. Nylon 6,10 is made from hexamethylene diamine (the diamine) and sebacoyl chloride (the diacid chloride).
SYNTHESIS OF NYLON 6,10. Hexamethylene diamine. Sebacoyl chloride.
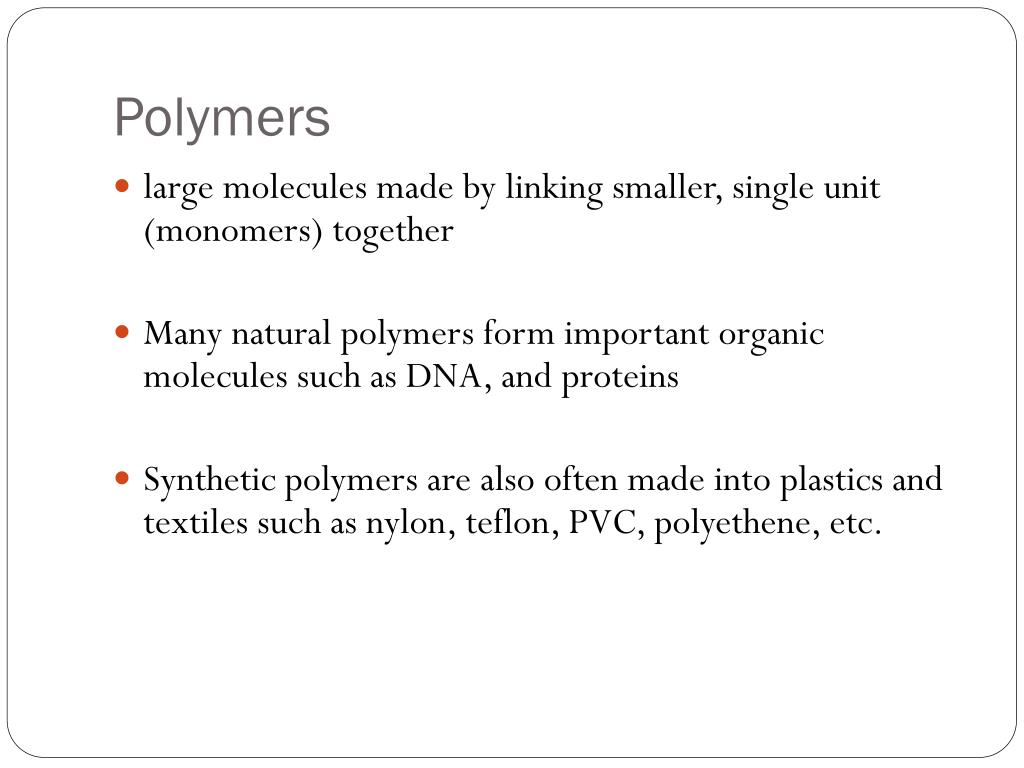
Synthetic polymers are classified by their method of synthesis. Synthetic Method. Chain-growth. Step-growth. polystyrene. Polyamides (nylon)
Proteins. hair, skin, tissue. Polysaccharides. cellulose, starch. Polynucleotides. DNA, RNA.
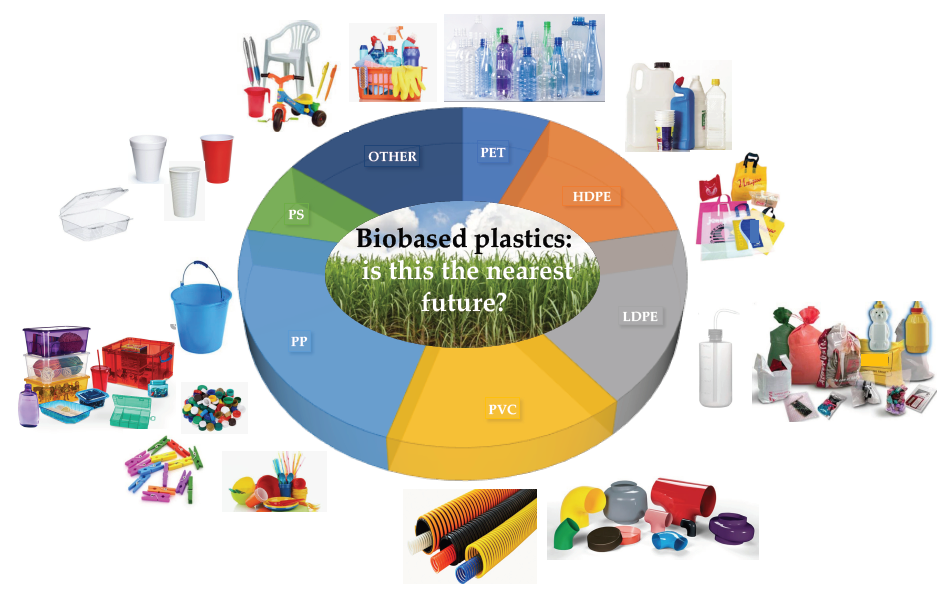
Nylons. Polyesters. Acrylics. Polyvinyls. Plastic sheeting and plumbing materials. Polystyrenes. Insulating materials.
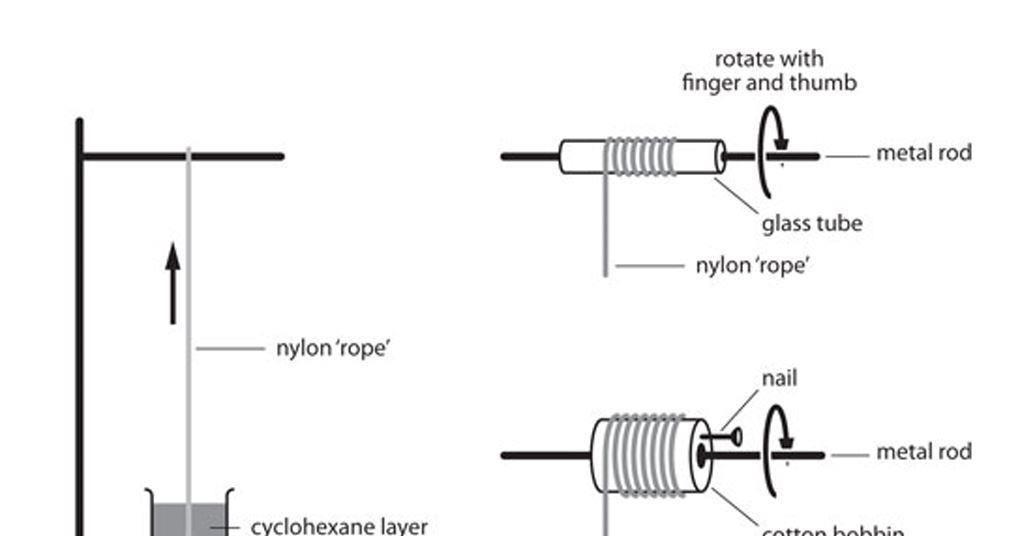
Pour diamine solution and diacid chloride in separate beakers. Using forceps, grasp the film that forms at the interface of the two liquids. Pull up slowly and wrap end of nylon string around a test tube. Rotate the tube and count the number of revolutions made before no more nylon can be produced.
Calculate the length of the nylon string produced (in meters) using the following equation: Nylon produced (m) = (test tube diameter (m)) X (p) X (# test tube revolutions )
Table Test tube diameter (mm) # of test tube revolutions. Length of nylon (m)
All chemicals are hazardous to the skin and eyes. Safety goggles and gloves are required during the experiment!!!
ORGANIC WASTE (Polymers) .
DO NOT return any glassware to lab drawer dirty or wet!
OBJECTIVE (Must clearly state…) What compounds will be made and how. What you will do with the compound once made. CHEMICAL EQUATION. Include the chemical equation middle of page 212. TABLE OF PHYSICAL DATA (Complete the following table using MSDS sheets from or ONLY. Wikipedia is unacceptable.) REFERENCE TO PROCEDURE (Must include…) full title including edition and author names. page numbers where actual procedure can be found. Compound. MW (g/mol) mp(oC) bp(oC) d (g/mL) HAZARDS. Sebacoyl chloride. X. Hexamethylene diamine. Sodium hydroxide. Hexane.
DATA/CALCULATIONS. Diameter of test tube. # revolutions. Length of nylon produced calculation. EXPERIMENTAL PROCEDURE. In paragraph form, BRIEFLY describe the procedure that you actually followed during the lab. Paragraph must be written in PAST TENSE, PASSIVE VOICE. Include any volumes or weights of chemicals used during the experiment. Include any mistakes, accidents or observations if applicable.

POLYMER- LECTURE-12) Synthesis and Applications of Synthetic Fiber- Nylon 6 By Dr. Nisha Singh
Polymers, Free Full-Text
Enzyme Immobilization: Method & Application
3. Manufacturing: Materials and Processing, Polymer Science and Engineering: The Shifting Research Frontiers

Polymers Notes: Meaning, types of Polymers & Polymerizations
Making nylon: the 'nylon rope trick', Experiment

Advancements in eco-friendly food packaging through nanocomposites: a review

Synthesis of renewable nylon monomers with poplar wood - ScienceDirect

HD WAM 91 Usyd Chemistry Notes 1A, CHEM1911 - Chemistry 1A (Advanced) - USYD
PPT - Polymers PowerPoint Presentation, free download - ID:1869894

Monomers & Polymers, Definition, Difference & Examples - Lesson

ORGANIC POLYMERS: THE SYNTHESIS OF NYLON - ppt video online download

PPT – Isomers and polymers PowerPoint presentation

PPT - Polymers and Polymerization PowerPoint Presentation, free download - ID:1835987